The Cancer Center/Regenerative Medicine Laboratory performs translational development of novel cell engineering methods leading to clinical production of novel therapies across a wide range of applications.
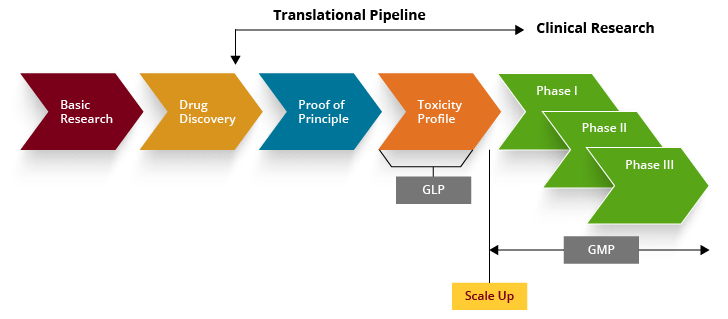
The general strategy in the development of a novel cellular therapeutics
Typically, a new potential cellular therapy, emanating from discoveries in a basic research laboratory, is first tested for "proof of principal" in relevant in vitro and in vivo model systems and other appropriate models for cell distribution and toxicology. With sufficient preclinical data demonstrating safety and efficacy in relevant animal models, large-scale product manufacturing schemes are developed and production methods are tested. After cGMP manufacturing methods are validated, the clinical trial can be initiated.